Deceased organ and tissue donation after medical assistance in dying: 2023 updated guidance for policy

Abstract
Background: Since Canadian Blood Services (CBS) developed policy guidance in 2019 for organ and tissue donation after medical assistance in dying (MAiD), the federal government has made changes to legislation related to MAiD. This document provides updated guidance for clinicians, organ donation organizations, end-of-life care experts, MAiD providers and policy-makers on the impact of these changes.
Methods: Canadian Blood Services assembled a group of 63 experts from critical care, organ and tissue donation, health care administration, MAiD, bioethics, law and research to review the legislative changes in the Organ and Tissue Donation After Medical Assistance in Dying – Guidance for Policy forum. Two patients who had requested and been found eligible for MAiD and 2 family members of patients who had donated organs after MAiD were also included as participants. In a series of 3 online meetings from June 2021 to April 2022, forum participants addressed a variety of topics in small and large groups. These discussions were informed by a comprehensive scoping review using JBI methodology. We used an adapted form of nominal group technique to develop the recommendations, which were approved by consensus of the participants. Management of competing interests was in accordance with Guideline International Network principles.
Recommendations: Although many of the recommendations from the guidance developed in 2019 are still relevant, this guidance provides 2 updated recommendations and 8 new recommendations in the following areas: referral to an organ donation organization, consent, directed and conditional donation, MAiD procedures, determination of death, health care professionals and reporting.
Interpretation: Policies and practices for organ and tissue donation after MAiD in Canada should align with current Canadian legislation. This updated guidance will help clinicians navigate the medical, legal and ethical challenges that arise when they support patients pursuing donation after MAiD.
The Supreme Court of Canada decriminalized medical assistance in dying (MAiD) in 2015 and Parliament passed Bill C-141 to outline the procedures and eligibility criteria for MAiD in Canada in June 2016.
In 2019, after multiple patients had asked to donate their organs and tissues after MAiD, Canadian Blood Services (CBS) developed policy guidance to serve as a foundation for clinicians, organ donation organizations, end-of-life care experts, MAiD providers and policy-makers to facilitate donation after MAiD, in the absence of any formal language in the legislation.2 Between 2016 and 2021, 155 patients donated their organs and tissues after MAiD in Canada.3
Subsequent to publication of the CBS initial guidance on donation after MAiD,2 the Quebec Superior Court found that 1 of the eligibility criteria outlined in Bill C-14 was incompatible4 with the original decision of the Supreme Court of Canada.1 Accordingly, the Minister of Justice and Attorney General of Canada introduced Bill C-7, adopted on Mar. 17, 2021, with changes to the following areas: eligibility criteria, safeguards, waiver of final consent and monitoring regime.5
The eligibility criteria were modified in Bill C-7 with removal of the “reasonably foreseeable natural death” criterion. Cases in which mental illness is the sole underlying medical condition have been temporarily excluded.4,5
Bill C-7 created 2 sets of safeguards: 1 set for patients whose natural death is reasonably foreseeable (Track 1 patients) and a second set of additional safeguards for patients whose natural death is not reasonably foreseeable (Track 2 patients), which includes a minimum 90-day assessment period.5
The potential to waive the requirement for final consent at the time of the MAiD procedure was introduced in Bill C-7 for Track 1 patients (those whose natural death is reasonably foreseeable and who were deemed eligible for MAiD), if they lose capacity to reaffirm consent before their scheduled date for MAiD and have a written arrangement with a practitioner.5
The reporting requirements were expanded in Bill C-7, based on experiences with the federal MAiD monitoring regime to date.5 As with Bill C-14, Bill C-7 does not comment on organ and tissue donation after MAiD.
To update its guidance for policy, CBS assembled a group of experts and patient and family partners to examine these legislative changes and their impact on donation after MAiD, in the Organ and Tissue Donation After Medical Assistance in Dying – Guidance for Policy forum. This document outlines the updated guidance and potential future work in this area.
Scope
The target audience for this updated guidance for policy includes clinicians, organ donation organizations, end-of-life care experts, MAiD providers and policy-makers.
The initial guidance2 was relevant to all patients who were conscious and competent and had made a decision leading to imminent death, including withdrawal of mechanical ventilation (invasive or noninvasive), withdrawal of extracorporeal support (including extracorporeal membrane oxygenation or other mechanical circulatory support) and MAiD (these patients are now referred to as “Track 1”).
This update focuses only on conscious, competent patients pursuing organ donation after MAiD. Now that patients whose natural death is not reasonably foreseeable are eligible for MAiD (“Track 2 patients”), this updated guidance applies to both Track 1 and Track 2 patients.
This guidance does not address the ethics of MAiD, questions regarding eligibility or assessment for MAiD, or provision of MAiD. It focuses on organ donation for those patients who have been assessed and found eligible for MAiD through established processes in Canada.
Recommendations
Many of the recommendations from the original guidance2 are still relevant. This current guidance provides 2 updated recommendations and 8 new recommendations, summarized in Box 1. Appendix 1 (available at www.cmaj.ca/lookup/doi/10.1503/cmaj.230108/tab-related-content) includes a summary table of original, new and updated recommendations.
Box 1: Summary of new and updated recommendations*
Referral to an organ donation organization
-
All Track 2 patients should be referred to the provincial organ donation organization for information-sharing if a patient initiates a discussion on donation, regardless of when this discussion occurs within the 90-day assessment period. (New recommendation)
Consent
-
Under circumstances in which a Track 1 patient has provided first-person consent for MAiD, including completion of a waiver of final consent and first-person consent for donation, but loses the capacity to reaffirm consent before death, first-person consent for donation should be upheld and next steps to facilitate donation should be coordinated with the SDM. (Updated recommendation)
-
Under circumstances in which a Track 1 patient has provided first-person consent for MAiD, including completion of a waiver of final consent, but loses capacity before first-person consent for donation, the SDM should be approached to 1) reaffirm consent for registered donors and those in jurisdictions with opt-out legislation, or 2) discuss and obtain consent for patients without registered consent if consistent with the patient’s wishes. (Updated recommendation)
-
All Track 2 patients who are potentially eligible for organ donation should be approached for first-person consent for donation after MAiD once MAiD eligibility has been confirmed, regardless of when their eligibility for MAiD is confirmed within the 90-day assessment period. (New recommendation)
Directed and conditional donation
-
Organ donation organizations and transplantation programs should develop a policy on directed deceased donation for patients pursuing MAiD, in alignment with the directed donation principles and practices that are in place for living donation in their jurisdiction. (New recommendation)
MAiD procedures
-
For Track 1 patients receiving MAiD after loss of capacity who require admission to the hospital for donation, transfer and admission to the hospital should be coordinated with the SDM. (New recommendation)
-
Track 2 patients must provide first-person consent immediately before the MAiD procedure. As such, first-person consent should be obtained before transfer and admission to hospital for donation. (New recommendation)
-
Further work is needed to assess the potential for donation after MAiD at home in Canada. In the interim, patient-initiated requests for donation after MAiD at home warrant consideration on a case-by-case basis, where feasible. (New recommendation)
Health care professionals
Reporting
-
Note: MAiD = medical assistance in dying, SDM = substitute decision-maker, Track 1 patients = patients whose natural death is reasonably foreseeable, Track 2 patients = patients whose natural death is not reasonably foreseeable.
-
↵* These recommendations replace or supplement recommendations from the original 2019 guidance.2 See Appendix 1 for a summary of all recommendations (original, new and updated).
Referral to an organ donation organization and consent (Track 2 patients)
All Track 2 patients should be referred to the provincial organ donation organization for information-sharing if a patient initiates a discussion on donation, regardless of when this discussion occurs within the 90-day assessment period. (New recommendation)
All Track 2 patients who are potentially eligible for organ donation should be approached for first-person consent for donation after MAiD once MAiD eligibility has been confirmed, regardless of when their eligibility for MAiD is confirmed within the 90-day assessment period. (New recommendation)
After receiving information on the new safeguards for Track 2 patients (i.e., those whose natural death is not reasonably foreseeable) that include a minimum 90-day assessment period, forum participants provided input as to when Track 2 patients should be approached about donation after MAiD. Preliminary discussion led to clarification of the difference between general information-sharing versus an approach for first-person consent for donation after MAiD.
Forum participants agreed that any Track 2 patient who expresses an interest in donation after MAiD should be referred to their provincial organ donation organization for information-sharing, regardless of when this occurs during the MAiD process. The provincial organ donation organization is responsible for assessing eligibility for organ donation.
Forum participants agreed that evaluation for organ donation suitability and obtaining first-person consent for donation after MAiD should be separate from and occur only after eligibility for MAiD has been confirmed. Most participants agreed that if MAiD eligibility is confirmed during the 90-day assessment period, then first-person consent for donation could occur during the 90-day assessment period (Figure 1). Some participants said they felt unable to comment given lack of experience with Track 2 patients. The participants discussed whether confirmation of MAiD eligibility required completion of the 90-day assessment period. However, they recognized that the 90-day assessment period is a safeguard, not an eligibility criterion. Typically, the provincial organ donation organization is responsible for approaching patients for consent to donation.
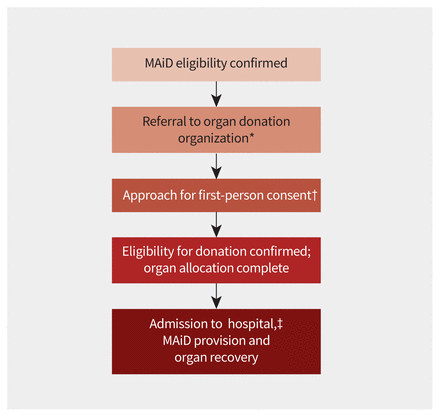

Flow chart for referral and consent for donation after medical assistance in dying (MAiD) in Track 2 patients. Note: SDM = substitute decision-maker, Track 2 patients = patients whose death is not reasonably foreseeable. *Referral to the provincial organ donation organization may occur before confirmation of MAiD eligibility after patient-initiated inquiry for purposes of information-sharing. †MAiD eligibility must be confirmed before approach. Approach and consent may occur during, or after, the mandatory 90-day assessment period. ‡In certain jurisdictions, donation after MAiD at home may be supported.
The supporting rationales for these recommendations included the following points. Approaching patients for first-person consent for donation late in the MAiD process involved potential risks, including insufficient time to facilitate donation. Obtaining first-person consent for donation after MAiD during the 90-day assessment period is similar to obtaining consent during the previously legislated 10-day reflection period. Family participants expressed strong support for approaching all patients pursuing MAiD, to facilitate adequate time for discussion and consideration of donation after MAiD. Patients should be fully informed regarding all options at end of life, including donation; and if patients are competent to make a decision regarding MAiD, they are competent to make a decision regarding donation after MAiD.
Consent (Track 1 patients)
Under circumstances in which a Track 1 patient has provided first-person consent for MAiD, including completion of a waiver of final consent and first-person consent for donation, but loses the capacity to reaffirm consent before death, first-person consent for donation should be upheld and next steps to facilitate donation should be coordinated with the substitute decision-maker (SDM). (Updated recommendation)
Under circumstances in which a Track 1 patient has provided first-person consent for MAiD, including completion of a waiver of final consent, but loses capacity before first-person consent for donation, the SDM should be approached to 1) reaffirm consent for registered donors and those in jurisdictions with opt-out legislation, or 2) discuss and obtain consent for patients without registered consent if consistent with the patient’s wishes. (Updated recommendation)
Forum participants were informed of the process of a waiver of final consent for Track 1 patients5 (i.e., those whose natural death is reasonably foreseeable) and discussed 2 approaches for circumstances in which a Track 1 patient loses capacity to consent after providing first-person consent for MAiD and donation: upholding first-person consent and reaffirming consent with the SDM.
The participants came to a consensus regarding upholding first-person consent, with strong support from the patient and family participants (Figure 2). This was aligned with first-person consent being the gold standard for decision-making in health care.6,7
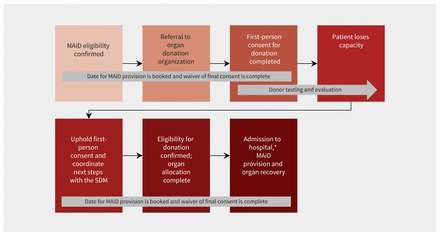

Flow chart for loss of capacity after first-person consent for medical assistance in dying (MAiD) and donation in Track 1 patients. Note: SDM = substitute decision-maker, Track 1 patients = patients whose natural death is reasonably foreseeable. *In certain jurisdictions, donation after MAiD at home may be supported.
Additional rationales in support of upholding first-person consent included honouring the patient’s wishes; upholding consent being potentially less burdensome for the SDM, as the decision has already been made by the patient; and the role of the SDM being to fulfill the patient’s wishes.6
If a Track 1 patient loses capacity after first-person consent for MAiD but before first-person consent for donation, the forum participants agreed that jurisdictional policy for donation after death determination by circulatory criteria8 should be followed, and the SDM should be approached to either reaffirm registered consent or obtain consent for patients without prior registration (Figure 3). The recommendations in the 2006 death determination guideline were updated in 2023.8
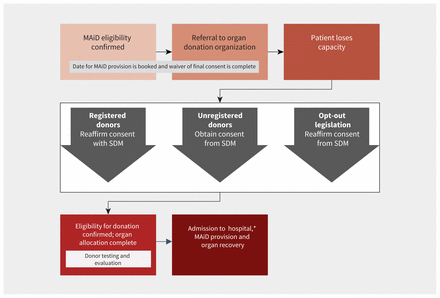

Flow chart for loss of capacity after first-person consent for medical assistance in dying (MAiD) but before first-person consent for donation in Track 1 patients. Note: SDM = substitute decision-maker, Track 1 patients = patients whose natural death is reasonably foreseeable. *In certain jurisdictions, donation after MAiD at home may be supported.
Given the January 2021 legislation change to “deemed consent” in Nova Scotia,9 where patients are assumed to have given their consent for deceased organ donation unless they have explicitly registered otherwise, forum participants discussed the approach for jurisdictions with this type of “opt-out” legislation and agreed that presumed consent should be reaffirmed with the SDM. Typically, the provincial organ donation organization is responsible for approaching patients or their SDM for consent to donation.
Directed and conditional donation
Organ donation organizations and transplantation programs should develop a policy on directed deceased donation for patients pursuing MAiD, in alignment with the directed donation principles and practices that are in place for living donation in their jurisdiction. (New recommendation)
Directed donation refers to a patient’s desire to have 1 or more of their organs given to someone known to them (a friend or relative) who needs an organ transplant.10 Organ donation organizations must confirm blood and tissue type matching. In recognition that some patients pursuing donation after MAiD request directed donation, forum participants agreed that organ donation organizations and transplantation programs should be prepared by developing relevant policies related to directed deceased donation after MAiD.
After a presentation on the international experience to date, forum participants discussed key aspects of directed deceased donation after MAiD. These included considerations of patient requests, unconditional consent and recipient eligibility. After discussion and review, the participants reached consensus on core principles. Box 2 lists these core principles intended to aid organ donation organizations and transplantation programs in the development of policies regarding directed deceased donation after MAiD.
Box 2: Principles for consideration in the development of policies for directed deceased donation after medical assistance in dying
General principles
-
Living donation, which takes place when a living person donates an organ (or part of an organ) for transplantation to another person, before death from patients considering medical assistance in dying (MAiD) should be neither offered nor encouraged. Should a patient insist on living donation before death, the request should be considered on a case-by-case basis.
-
Requests for directed deceased donation after MAiD should be considered on a case-by-case basis in a manner consistent with living–donor directed donation practices in their jurisdiction.
-
Assessment of eligibility for MAiD must happen before any discussion about donation and eligibility for donation must be established before any discussion of directed deceased donation.
Patient request considerations
-
A patient’s request for directed deceased donation after MAiD must be made voluntarily.
-
Directed donation should not proceed if there is indication of monetary exchange or similar valuable consideration or coercion involved in the decision to pursue directed donation.
-
The intended recipient in a directed deceased donation case should be a family member or “close friend” — an individual with whom the donor or donor’s family has had a long-standing emotional relationship. This practice is consistent with living donation, in which the specified recipient is most often closely related to the donor.
Unconditional consent considerations
-
Consent for directed donation after MAiD will not be considered in cases where a patient or the substitute decision-maker places conditions on the member of a group, class or organization who should be in receipt of the organ if the intended donation cannot be realized.
-
In line with current Canadian guidance, the patient should be informed and understand they may withdraw consent for MAiD or donation at any time, and that withdrawal of consent for donation does not affect their consent for or access to MAiD.
Recipient eligibility considerations
-
The intended recipient must be on the current transplant waiting list or meet criteria for the same.
-
The recipient in greatest need will be prioritized, even if that is not the intended recipient.
-
Transplantation will proceed only if the donor organ is medically compatible with the intended recipient.
-
The intended recipient should be informed and understand that they may withdraw consent for transplantation at any time and that withdrawal of consent does not affect their position on the wait-list.
MAiD procedures
For Track 1 patients receiving MAiD after loss of capacity who require admission to the hospital for donation, transfer and admission to the hospital should be coordinated with the SDM. (New recommendation)
Track 2 patients must provide first-person consent immediately before the MAiD procedure. As such, first-person consent should be obtained before transfer and admission to hospital for donation. (New recommendation)
Further work is needed to assess the potential for donation after MAiD at home in Canada. In the interim, patient-initiated requests for donation after MAiD at home warrant consideration on a case-by-case basis, where feasible. (New recommendation)
A scoping review commissioned for this project found that MAiD provisions, with rare exceptions, occur primarily in hospital when donation occurs.11–13 Given the different consent pathways for Track 1 and Track 2 patients, the coordination of admission to hospital may vary. By law, Track 2 patients must provide consent for MAiD at the time of the procedure, and first-person consent must thus be confirmed before transfer to hospital. Track 1 patients can receive MAiD after loss of capacity via a waiver of final consent. For Track 1 patients with loss of capacity, transfer should be coordinated with their SDM.
The potential benefits of admission to hospital for donation after MAiD include improved control over the patient’s health condition, timely transfer to the operating room, reduced warm ischemia time and easier access to services and resources.14–16 However, the requirement to receive MAiD in hospital may be a deterrent for patients who wish to die at home. Organizations in both Canada and the Netherlands have been able to accommodate patients’ wishes to die at home and pursue donation after MAiD.14,15,17
In a case in the Netherlands, the patient’s family physician administered a sedative while an anesthesiologist–intensivist waited out of sight. When the patient became nonresponsive and family members were ready, the anesthesiologist–intensivist administered anesthesia and endotracheal intubation, before the patient was transferred to the hospital via ambulance. In the hospital, the family physician performed the MAiD procedure and confirmed death before organ recovery.14,15
In a case in Ontario, the patient received the MAiD procedure at home.16 When the family was ready, the patient was transferred to an ambulance, where death was confirmed. Intubation was performed and an orogastric tube was secured before the patient was placed prone on the ambulance stretcher. The rest of the protocol for lung protection during the absence of circulation was applied before the patient was transferred to the hospital for organ recovery.17
Most forum participants agreed that donation after MAiD at home should be offered in Canada. Some of the participants were unsure and 1 disagreed, given concerns regarding negative memories for loved ones. Some were uncertain about the feasibility of donation after MAiD at home in many jurisdictions. Not all jurisdictions have a nonperfused organ donation protocol like that used in Ontario, and it is unclear whether the protocols used in the Netherlands would be allowable in Canada.
Further work is necessary to assess the potential for a medical, ethical and legal framework for donation after MAiD at home in the Canadian context. In the interim, patient-initiated requests for donation after MAiD at home warrant consideration on a case-by-case basis where feasible, so that opportunities to fulfill a patient’s end-of-life wishes are not missed.
Health care professionals
Health care professionals involved in donation after MAiD require specialized education, training and support. (New recommendation)
Donation after MAiD involves a wide range of health care professionals: family physicians, MAiD assessors and providers, donation coordinators, organ donation organizations and hospital teams involved in the donor’s suitability testing, screening and organ recovery.
For donation coordinators, working with conscious, competent patients who are considering donation at the end of life, is a unique experience. Given the longer time frame for donation after MAiD compared with a typical donation case and the requirement for first-person consent, a stronger relationship can develop between the coordinator and the patient and their family.18 These bonds may make it difficult for coordinators to witness the patient’s death, adding to their experience of grief and loss.18 Working directly with patients (versus with SDMs) appears to add additional pressure, whether real or perceived, to ensure all questions are adequately answered, the logistics of suitability testing and screening happen seamlessly (despite being more complex), and any delays in MAiD related to donation are avoided.18
Forum participants agreed that all health care professionals involved in donation after MAiD require specialized education and training. This should include standardized training on MAiD legislation, donation after MAiD processes and first-person consent, and roles and responsibilities of all members of the end-of-life care and donation teams.
Forum participants also agreed on the need for enhanced support for organ donation coordinators, including interprofessional collaboration; increasing the number of coordinators assigned to MAiD cases; focused sessions on resilience, grief and loss; and after-care support, including case debriefing and access to employee assistance programs.
Currently, education of health care professionals is the responsibility of each provincial jurisdiction or organ donation organization.
Reporting
Efforts to formalize the collection and reporting of data specific to donation after MAiD should be prioritized by organ donation organizations. (New recommendation)
Currently, there is provincial variability in the data collection for donation after MAiD. Information provided by forum participants showed that most provinces participating in donation after MAiD are collecting the number of patients referred for donation, patients who consent to donation, and patients donating organs.
Forum participants agreed that collecting and reporting data specific to donation after MAiD is an important aspect of performance measurement and required for continued improvement and future strategy development. The suggested data include the number of patients who undergo MAiD and are referred for donation, who are eligible for donation, who were approached for donation and who consented to donation, as well as patients donating organs and organs transplanted per donor.
To normalize data collection and reporting of donation after MAiD, forum participants suggested data be reported publicly and collected in the same manner that donation data are collected now: through CBS, the Canadian Institute for Health Information and Canada Health Infoway.
Currently, funding for data collection is the responsibility of each provincial jurisdiction or organ donation organization.
Methods
Canadian Blood Services organized and funded the development of this updated guidance in response to the changes to Bill C-7 legislation4 in 2021 and requests for guidance from the community. Canadian Blood Services is a national, not-for-profit charitable organization that manages the supply of blood and blood products in all provinces and territories in Canada (except for Quebec). It is responsible for national activities related to organ and tissue donation and transplantation, which includes national system development and operation of interprovincial organ sharing programs.
In a series of online meetings, a group of experts used a modified version of nominal group technique18 to develop the agreed-upon recommendations. Before the meetings, the planning committee commissioned a scoping review11,12,19,20 of relevant literature and assembled a variety of background documents to inform the discussions.
Composition of participating groups
The System Development team at CBS established a 9-member planning committee (including K.L., C.M., S.S. and L.W., co-chaired by K.W. and J.D.) to initiate and design the guidance development process. The committee engaged 63 experts from relevant fields — including critical care, organ and tissue donation, health care administration, MAiD, bioethics, law and research — to participate in the guidance development process. Participants were selected according to their specialty, expertise, professional society representation and geographic diversity.
From project onset, we determined that the involvement and perspectives of patient partners would be critically important. The committee also invited the participation of 2 patients who had requested and been found eligible for MAiD and 2 family members of patients who had donated organs after MAiD.
Formal representation from professional societies included the Canadian Donation and Transplantation Research Program, the Canadian Society of Transplantation, the Canadian Association of MAiD Assessors and Providers and the Canadian Association of Critical Care Nurses. The committee also invited 2 international experts who investigated or had been involved in organ donation after MAiD at home in the Netherlands and Belgium to participate.
The committee assigned a 3-person “Listening for Research” panel with expertise in intensive care, organ donation, transplantation and research to collect knowledge gaps and research opportunities identified throughout this guidance development process.
A complete list of forum participants, planning committee members and members of the “Listening for Research” panel with their affiliations is available in Appendix 2 (available at www.cmaj.ca/lookup/doi/10.1503/cmaj.230108/tab-related-content).
Selection of priority topics
The committee defined the scope of this updated guidance based on the changes to legislation and community-derived requests for guidance.
In response to the legislation, the forum sought to examine the impacts of Track 1 patients’ loss of capacity and use of waiver of final consent for MAiD on consent for organ donation, and when to approach Track 2 patients for consent to donate, given the new requirement for a 90-day assessment period.
In the months leading up to the forum, CBS had received requests from clinicians and administrators of organ donation organizations to revisit the recommendations for directed donation after MAiD that had been included in the original guidance,2 as well as to explore the acceptability and feasibility of organ donation after MAiD at home.
We added national data collection, health care professional education and knowledge gaps to the agenda to help inform future strategy development at CBS.
Literature review
Before the meetings, the planning committee commissioned a scoping review of the literature relevant to donation after MAiD.11–13 The scoping review was conducted by a research team with expertise in literature reviews, using the JBI methodology.21,22 To search for published literature, a 3-step search strategy was developed and implemented by an information specialist under the guidance of the research team and using the peer review of electronic search strategies (PRESS) guideline statement process.19
The search was conducted in March 2021 and updated in December 2021 using MEDLINE, Embase, CINAHL, PsycINFO, Web of Science (Science Citation Index and Social Science Citation Index) and Academic Search Complete, with no studies excluded based on language.21 The information specialist requested grey literature and unpublished materials from key stakeholders and conducted searches for related information via Google and websites of organ donation organizations from countries where patients requesting MAiD could also request organ donation. Reports published from 2000 and onward in any language, country and research design were considered.
Development of recommendations
Canadian Blood Services hosted a 2-day forum on June 21 and 23, 2021, as well as a 2-hour supplementary meeting on Apr. 6, 2022, for all participants. All meetings were conducted online.
We provided the preliminary findings of the scoping review11–13 to forum participants in advance of the first meeting, in addition to background documents that were developed by the planning committee to guide and support the discussion. These documents included an overview of the legislation changes, proposed clinical pathways for discussion and relevant published literature1,2,4,20 (Appendix 3, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.230108/tab-related-content). At the meetings, forum participants also heard presentations from national and international experts, as well as patient and family partners, to inform the discussion.
We generated recommendations using a modified version of nominal group technique,23 a structured variation of small-group discussion to reach consensus. Forum participants were divided into small groups ranging from 10 to 14 people (including the facilitators); the group’s moderator presented specific questions to be addressed for each topic. Group members took turns sharing their initial perspectives, all of which the moderators recorded in real time on a shared screen. Each group discussed the logged ideas to achieve consensus. Participants then reconvened in plenary, where key ideas and considerations from each group were presented and discussed to achieve consensus among all participants. There was no formal voting process for the final recommendations.
We held a second meeting to discuss the approach for consent for donation after MAiD for Track 2 patients, a gap identified by participants and the planning committee after completion of the initial meeting.
After the meetings, the planning committee drafted a summary report that included the agreed-upon recommendations. Before the report was finalized, the committee emailed it to participants to review for accuracy.
The core principles listed in Box 2 help support organ donation organizations and transplantation programs in the development of policies on directed deceased donation after MAiD. We developed these principles in the same manner as the recommendations. To support discussion and decision-making regarding these principles, we provided participants with the Directed Donation Clinical Process Instruction from Trillium Gift of Life Network (Ontario’s organ donation organization).22 Consensus on the final list of principles was achieved using a modified version of nominal group technique16 described above.
The findings of the “Listening for Research” panel on knowledge gaps and research opportunities raised during forum discussions, along with those identified by the scoping review,11–13 were informally categorized (i.e., formal qualitative methodology was not used) into themes by the Listening for Research panel members, as well as L.W. and K.L.
Management of competing interests
The management of competing interests was in accordance with Guideline International Network principles.24 Before their participation, all planning committee members and forum participants were required to complete a Canadian Blood Services’ disclosure of competing interests form to declare any real, potential or perceived competing interests (Appendix 4, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.230108/tab-related-content). A team from CBS staff (L.W., K.L., S.D.) reviewed and managed these forms. Several forum participants had professional roles in organ donation administration; however, no forum participant was deemed to have a competing interest that prohibited their participation.
Canadian Blood Services funded and facilitated the recommendation generation process. However, the meeting participants developed and approved the recommendations.
Implementation
To facilitate national adoption of the recommendations, this guidance document will be distributed to relevant stakeholders, particularly the transplantation programs of organ donation organizations, as well as intensive care and MAiD provider communities across Canada.
Given the variation in practices relating to both MAiD and donation after MAiD across Canada, some jurisdictions may be unable to apply the updated guidance. Specifically, in jurisdictions reliant on patient initiation of donation after MAiD, lack of awareness of the option may result in missed opportunities. Jurisdictions without central coordination of MAiD may experience similar challenges. There are also jurisdictional variations in the education, training and support provided to coordinators who facilitate donation after MAiD.
Provincial jurisdictions or organ donation organizations are responsible for the implementation and monitoring of the recommendations in this guideline. However, as the organization responsible for national activities related to organ and tissue donation and transplantation, CBS will continue to review and monitor changes to MAiD legislation (e.g., eligibility criteria for MAiD) as well as patient- and community-derived requests to determine whether any new medical, legal, ethical or logistical concerns warrant further updates to this guidance.
Other guidelines
As noted by Silva e Silva and colleagues, guidance and protocols for organ donation after MAiD exist in 3 different countries (Canada, Belgium and the Netherlands).11 Guidance among these countries differs slightly, but overall includes aspects of eligibility, referral processes, consent, mental health support for professionals, quality improvement tools, and safeguards and guidance for patients and health care professionals.11
Gaps in knowledge
International experience with donation after MAiD is limited. As noted earlier, we organized the knowledge gaps and research opportunities identified by the “Listening for Research” panel and in the scoping review11–13 into themes. These were public perception and understanding of donation after MAiD; system processes and procedures; experiences of health care professionals, patients and families; system data; implications for transplantation; and donation after MAiD at home.11–13 A complete list of the knowledge gaps and research opportunities identified is available in Appendix 3. Clinician scientists and relevant stakeholders are encouraged to explore research opportunities in the field to address these gaps in knowledge.
Limitations
As noted above, international experience with donation after MAiD is limited, and therefore, we found limited data to inform our recommendations. There was potential bias among forum participants, given that they were generally supporters of the current deceased donation and transplantation system, as well as donation after MAiD. Not all invitees were able to attend the forum; as such, this updated guidance may lack full national representation. We did not consider diversity of participants when canvassing for participation. Most participants were from the donation community, with potential under-representation of the MAiD assessor and provider perspectives. Participants also had variable and limited experience with donation after MAiD for Track 2 patients.
The planning committee formulated the various tools used to direct discussions and inform the final recommendations, with no input from participants. More fulsome discussions were limited because of forum time constraints. Finally, the manuscript was not reviewed by external stakeholders before submission for publication.
Conclusion
The purpose of this updated guidance is to continue to inform the development of policies and practices of donation after MAiD. This will help clinicians navigate the medical, legal and ethical challenges that arise when they support patients pursuing donation after MAiD.
Acknowledgements
This guidance was developed on behalf of Canadian Blood Services in collaboration with the Canadian Society of Transplantation, the Canadian Association of Critical Care Nurses, the Canadian Donation and Transplantation Research Program, and the Canadian Association of MAiD Assessors and Providers. The authors thank Lee James and Fiona Slater for their contribution to the conception of the updated guidance for policy project. The planning committee thanks all participants who contributed to the Organ and Tissue Donation after Medical Assistance in Dying forum. The development of this guideline would not have been possible without their contributions. A special thanks goes out to the patient and family partners (including Nichole Riese, Sandra Wadsworth and Randy Tresidder), whose experiential knowledge was openly shared to inform discussions and development of this guidance. Their perspectives kept the meetings focused on the overarching purpose that drove this initiative: providing high-quality end-of-life care and donation options for dying patients while protecting their interests, supporting grieving families and improving transplant opportunities for patients on wait-lists.
Footnotes
-
Competing interests: Lindsay Wilson, Ken Lotherington and Caitlin Mills are full-time, paid employees of Canadian Blood Services (CBS). Sam Shemie reports earning salary support as a medical advisor, system development, from CBS. Kim Wiebe reports that CBS provided writing support and article processing charges, and CBS provided support for Dr. Wiebe to attend its Organ and Tissue Donation and Transplant educational event in February 2023. No other competing interests were declared.
-
This article has been peer reviewed.
-
Contributors: Kim Wiebe, James Downar, Sam Shemie, Ken Lotherington, Lindsay Wilson and Caitlin Mills contributed to the conception and design of the work. All of the authors contributed to the design and conduct of the guidance development meetings. All of the authors drafted the manuscript, revised it critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
-
Funding: This work was financially supported by Canadian Blood Services. Canadian Blood Services receives funding from the provincial and territorial Ministries of Health and the federal government, through Health Canada. The views expressed herein do not necessarily represent the views of the federal, provincial or territorial governments. Canadian Blood Services is not responsible for the management or funding of any Canadian organ donation organization or transplant program.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See:
link